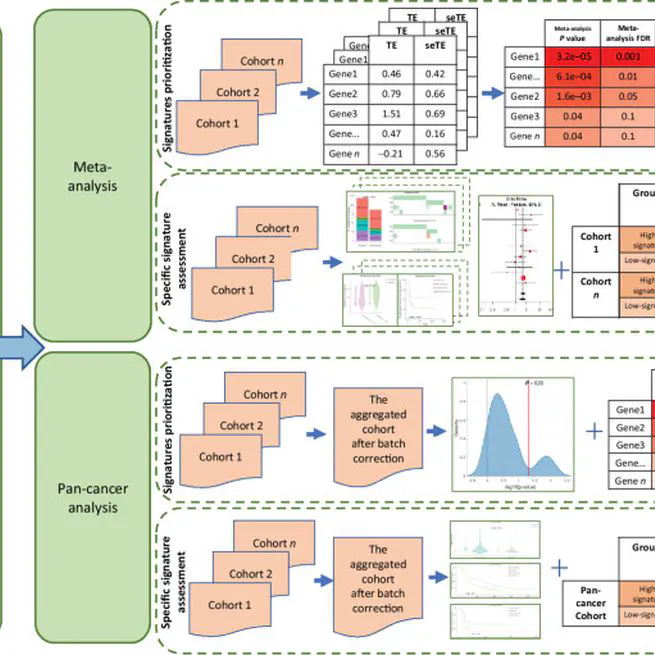
A pan-cancer immunogenomic atlas for immune checkpoint blockade immunotherapy
Dec 15, 2021
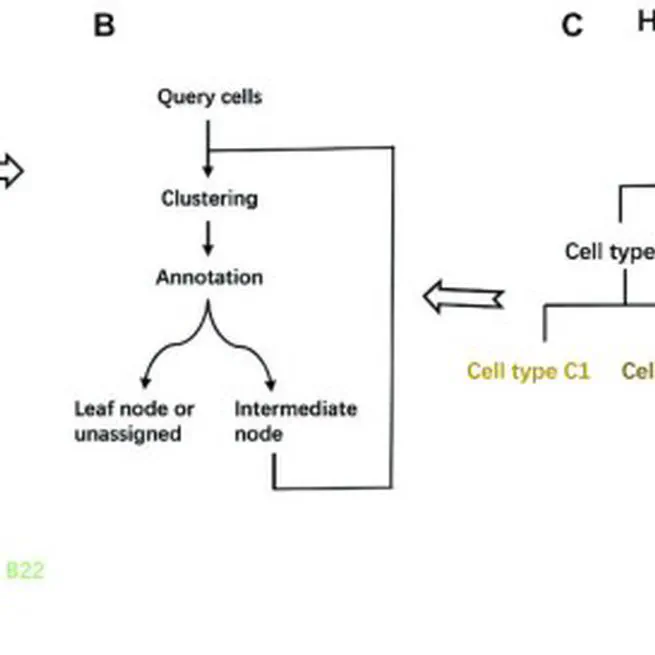
cell identities prediction for single-cell data
Oct 15, 2021
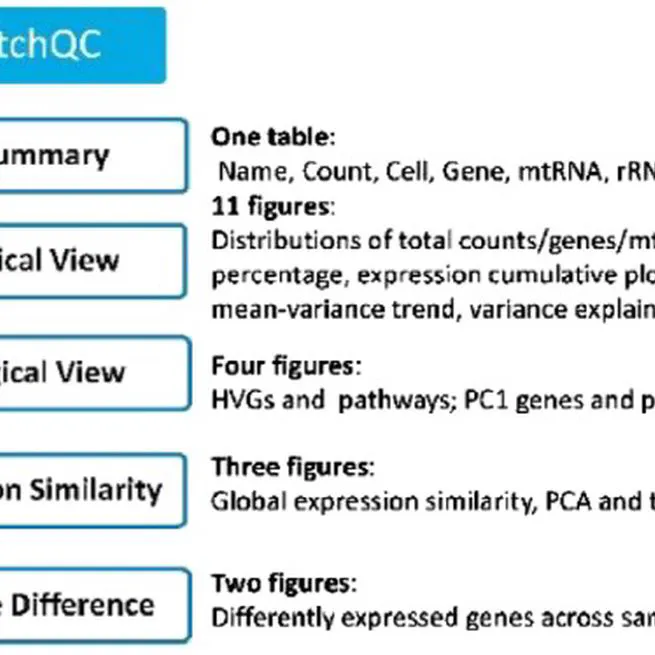
batch quality control for single-cell transcriptome
Aug 3, 2019
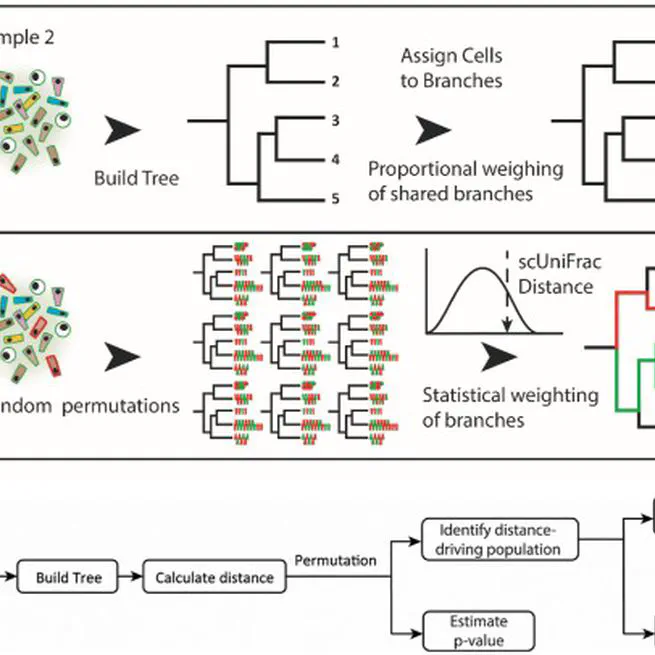
Abstract Single-cell RNA sequencing (scRNA-seq) has become a powerful tool for the systematic investigation of cellular diversity. As a number of computational tools have been developed to identify and visualize cell populations within a single scRNA-seq dataset, there is a need for methods to quantitatively and statistically define proportional shifts in cell population structures across datasets, such as expansion or shrinkage or emergence or disappearance of cell populations. Here we present sc-UniFrac, a framework to statistically quantify compositional diversity in cell populations between single-cell transcriptome landscapes. sc-UniFrac enables sensitive and robust quantification in simulated and experimental datasets in terms of both population identity and quantity. We have demonstrated the utility of sc-UniFrac in multiple applications, including assessment of biological and technical replicates, classification of tissue phenotypes and regional specification, identification and definition of altered cell infiltrates in tumorigenesis, and benchmarking batch-correction tools. sc-UniFrac provides a framework for quantifying diversity or alterations in cell populations across conditions and has broad utility for gaining insight into tissue-level perturbations at the single-cell resolution.
Oct 23, 2018
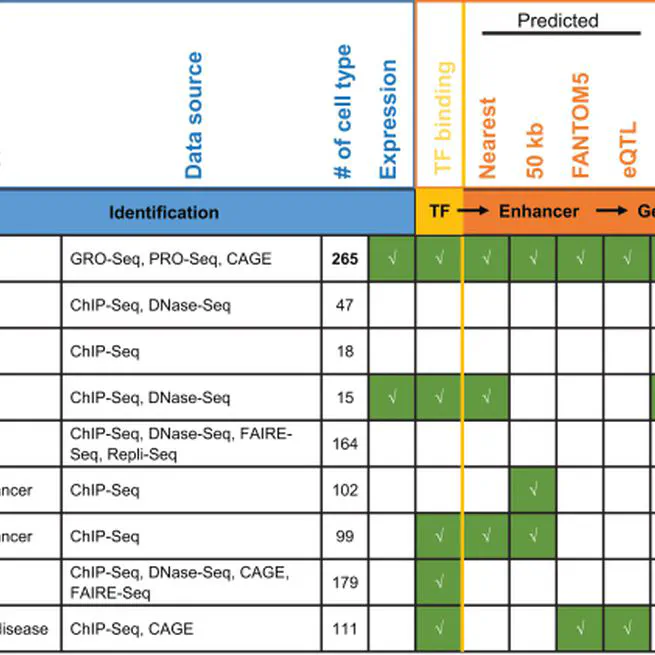
human enhancer database to interpret regulatory variants
Sep 25, 2018